Since I will discuss energy in many posts, it is worth spending a little time on units.
The Zoo of Units
Using meters, kilograms, and seconds (MKS) as the baseline, the natural unit that pops out is Joules (J). One Joule is the same as a kilogram times meters-per-second squared. Want a way to remember this? Try E = mc²: mass times velocity squared. Likewise, the kinetic energy of an object is ½mv²: same units.
But one Joule is a small amount of energy. When an apple falls off a table, it hits the floor with about one Joule of energy. So we often use bigger units like Btu, kilocalorie, kilowatt-hour, etc.
Before presenting conversions between units, we would do well to also understand power. Power is nothing more than the rate at which energy is used. The familiar unit, the Watt, is simply Joules per second. A 100 W incandescent light bulb is spewing 100 J of energy per second in the form of light and heat. It does not make sense to talk of Watts per second or Watts per hour. Watts is already a speed of energy consumption, not an energy unit on its own. I like to think of power as being like what your speedometer tells you: the rate at which you’re ticking off distance. The odometer is then the energy analog: how much distance you’ve accumulated. Or if you think of energy as being like money, then power is the rate at which you spend (or make) money: like a rent or a salary.
We cover power here so that the widely used unit of energy called the kilowatt-hour can be understood. This is not kilowatts per hour, but kilowatts times hours. A kilowatt-hour is the amount of energy accumulated by running something at 1000 W for one hour (3600 seconds). Or it could be 100 watts for ten hours. In any case, it reduces to 3,600,000 J (e.g., 1 kW times 1 hr is equivalent to 1000 J/s times 3600 seconds).
Now let’s get to the point of comparing units.
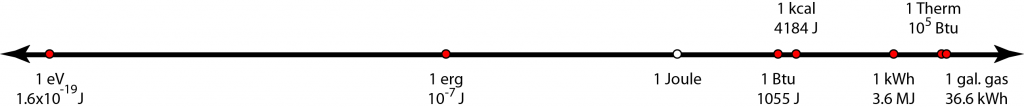
The figure above lays out energy units on a logarithmic scale, covering about 27 orders of magnitude. Memorizing these numbers will allow you to convert arbitrarily between units as needed. I carry these around in my head wherever I go, and it’s surprising how useful they are in interpreting newspaper articles, utility bills, hot water heater labels, and so on.
A few notes on each one:
- The electron-volt (1.6×10−19 J) is the amount of energy a single electric charge (electron, or proton for that matter) gains when crossing an electric potential of one volt. Light quanta (photons), thermal energy per particle, and chemical reaction energies per molecule are conveniently expressed in eV. For instance, visible light photons have energies around 1.8–3.1 eV. Thermal energy per particle (e.g., air molecule) at room temperature averages 1/40 eV. Chemical reactions per molecule tend to be in the 0.1–10 eV range.
- The erg is the metric unit for energy in CGS (centimeters, grams, seconds) and is 10 million times smaller than the Joule, in MKS.
- The Joule is the fundamental MKS unit of energy: kg·m²/s².
- The British thermal unit, Btu (1055 J), is very approximately 1 kJ. It is the amount of energy needed to raise the temperature of one pound of water by 1°F.
- The kilocalorie (4184 J), also denoted as Calorie with a capital C, is the amount of energy it takes to raise the temperature of one kilogram of water by 1°C. It is therefore a close analog of the Btu, but in the metric system. Our food energy is often expressed in kilocalories, and it is convenient to remember that carbohydrates, proteins, and fats contain 4, 4, and 9 kcal/g, respectively. It can also be useful to remember the fossil fuel values around 4–7, 10, and 13 kcal/g for coal, oil, and natural gas, respectively.
- The kilowatt-hour was discussed above, and in Joules is 1000 J/s × 3600 s = 3,600,000 J, or 3.6 MJ.
- The Therm is 100,000 Btu, or roughly 108 J. This is often used for natural gas metering, where one hundred cubic feet (hcf, or ccf) of natural gas has an energy content of 1.02 Therms. One Therm is 29.3 kWh.
- Though not an energy unit, I frequently want to know the energy content of a gallon of gasoline, and the number that sticks is 36.6 kWh.
Embrace the Power
While I have concentrated on energy units above, let me convince you that what we really usually want and express is power. Here are some familiar examples:
- You eat 2000 kcal/day. That’s 97 W.
- Your house used 306 kWh last month. That’s 400 W.
- The tag on the refrigerator says it will use 400 kWh per year. That’s 45 W.
- The hot water heater says it produces 30,000 Btu/hr. That’s 8800 W.
- Your natural gas bill is 30 Therms per month. That’s 1200 W.
- The U.S. generates about 4000 TWh of electricity per year. That’s 450 GW.
Truly, we often don’t care about the odometer (the police officer certainly doesn’t: “but officer, my odometer…”), or the total energy. We more often care about the rate: energy per time. And that’s power. This is why I claim my favorite unit (don’t you have one?) is the Watt. Now turn Abbot and Costello loose on that one…
Views: 21230
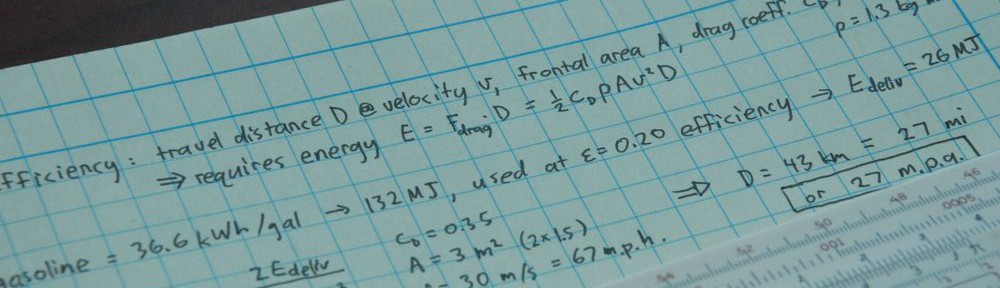
Hi Tom,
I somtimes find it useful to express large amounts of energy in terms of Megatons. Most people have an appreciation that a megaton nuclear explosion is a very big “boom” indeed. So here is the conversion I find useful.
1 Megaton =4.18E15 J.
So looking at your post on galactic-scale energy the current global power production is something like 1.2E13 watts. Since we have ~3.2E7 seconds in a year, this is 3.7E20 joules, or 90,533 Megatons a year! The point is we currently produce a lot of energy.
cheers
Geoff