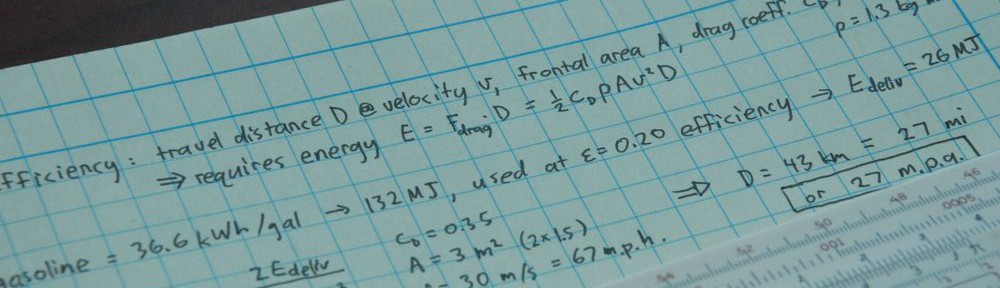We’ve all heard it. We think we understand it: entropy is a measure of disorder. Combined with the Second Law of Thermodynamics—that the total entropy of a closed system may never decrease—it seems we have a profound statement that the Universe is destined to become less ordered.
We’ve all heard it. We think we understand it: entropy is a measure of disorder. Combined with the Second Law of Thermodynamics—that the total entropy of a closed system may never decrease—it seems we have a profound statement that the Universe is destined to become less ordered.
The consequences are unsettling. Sure, the application of energy can reverse entropy locally, but if our society enters an energy-scarce regime, how can we maintain order? It makes intuitive sense: an energy-neglected infrastructure will rust and crumble. And the Second Law stands as a sentinel, unsympathetic to deniers of this fact.
A narrative has developed around this theme that we take in low entropy energy and emit a high entropy wake of waste. That life displays marvelous order—permitted by continuous feeding of this low entropy energy—while death and decay represent higher entropy end states. That we extract low entropy concentrations of materials (ores) from the ground, then disperse the contents around the world in a higher entropy arrangement. The Second Law warns that there is no going back: at least not without substantial infusion of energy.
But wait just a minute! The preceding paragraph is mostly wrong! An unfortunate conflation of the concepts of entropy and disorder has resulted in widespread misunderstanding of what thermodynamic entropy actually means. And if you want to invoke the gravitas of the Second Law of Thermodynamics, you’d better make darned sure you’re talking about thermodynamic entropy—whose connection to order is not as strong as you might be led to believe. Entropy can be quantified, in Joules per Kelvin. Let’s build from there.
Views: 5668